SEE STUDY RESULTS

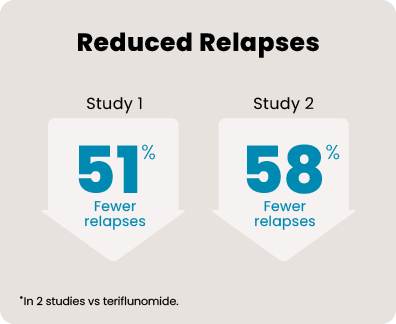
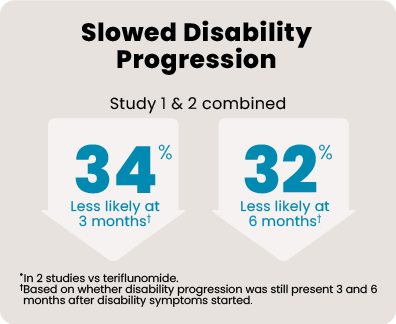
*In 2 studies vs teriflunomide.
Individual results may vary.
Results that Speak for Themselves
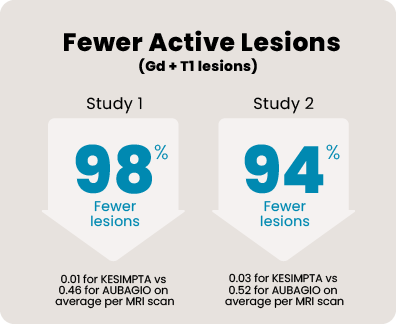
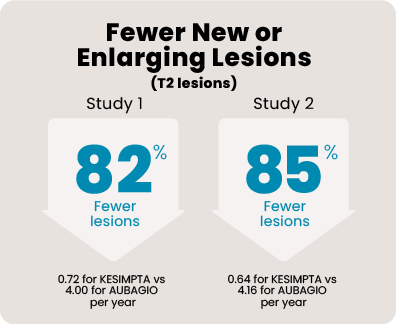
Over 1800 people aged 18 to 55, with relapsing MS, participated in 2 head-to-head studies that compared KESIMPTA to AUBAGIO® (teriflunomide). In the 1 to 2 years leading up to the studies, all of them had experienced a relapse or saw activity on an MRI scan. Each person received KESIMPTA 20 mg or AUBAGIO 14 mg for up to 30 months.
465 people received KESIMPTA in Study 1, and 481 people in Study 2. 462 people received AUBAGIO in Study 1, and 474 people in Study 2.

PROVEN EFFECTIVE*

MAGGIE S.
Mom, Recent Grad
Switched to KESIMPTA: 2021
“Since starting KESIMPTA, I've had fewer relapses, which is really cool. I love seeing that it's really working for me.”
Hear from people who take KESIMPTA:
The KESIMPTA Crew
The details are here for you
Want more information to help you decide?
Find out why so many people are choosing KESIMPTA.
Want co-pay help?
97% of all prescriptions filled have a $0 out of pocket cost when used with the Access Card‡§ from the Alongside™ KESIMPTA Patient Support Program.
All people shown on this webpage are living with relapsing multiple sclerosis, have taken KESIMPTA, and have been compensated for their time.
RMS, relapsing multiple sclerosis.
‡Limitations apply. Offer not valid under Medicare, Medicaid, or any other federal or state health insurance program. Patients with commercial insurance who are initially denied coverage may receive free KESIMPTA for up to 12 months while seeking coverage. Patients with commercial insurance who have coverage for KESIMPTA may receive up to $18,000 in annual co-pay benefits. Novartis reserves the right to rescind, revoke, or amend this program without notice. Additional limitations may apply. See complete Terms & Conditions at start.kesimpta.com.
§2023 data on file.